A monatomic gold reactor constructed in a conical biological nanopore
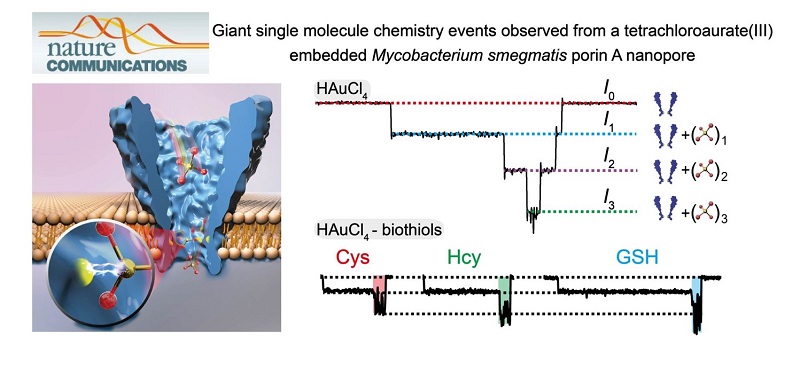
Image from Dr. Shuo Huang’s Lab. http://hysz.nju.edu.cn/bionano
Dr. Shuo Huang's team published the paper "Giant single molecule chemistry events observed from a tetrachloroaurate(III) embedded Mycobacterium smegmatis porin A nanopore" at Nature Communications in 11 Dec 2019. The plasmid α-HL M113G and MspA-M of this article have been deposited on MolecularCloud.
Biological nanopores are capable of resolving small analytes down to a monoatomic ion. In this research, tetrachloroaurate(III), a polyatomic ion, is discovered to bind to the methionine residue (M113) of a wild-type α-hemolysin by reversible Au(III)-thioether coordination. However, the cylindrical pore geometry of α-hemolysin generates shallow ionic binding events (~5-6 pA) and may have introduced other undesired interactions.
Inspired by nanopore sequencing, a Mycobacterium smegmatis porin A (MspA) nanopore, which possesses a conical pore geometry, is mutated to bind tetrachloroaurate(III). Subsequently, further amplified blockage events (up to ~55 pA) are observed, which report the largest single ion binding event from a nanopore measurement. By taking the embedded Au(III) as an atomic bridge, the MspA nanoporeis enabled to discriminate between different biothiols from single molecule readouts. These phenomena suggest that MspA is advantageous for single molecule chemistry investigations and has applications as a hybrid biological nanopore with atomic adaptors.
Plasmids from this article in MolecularCloud
| Cat. No. | Plasmid Name | Description | Price | Ordering |
|---|---|---|---|---|
| MC_0068792 | α-HL M113G | The plasmid α-HL M113G is designed for the preparation of the heptameric transmembrane protein alpha-hemolysin (α-HL) M113G by prokaryotic expression and to prove that methionine at site 113 is the key amino acid for gold ion binding. A H6 tag is designed for the followup purification using nickel affinity chromatography. Since 1996, α-HL WT along with its mutants have been widely investigated as single-molecule nanopore sensors, suitable for sensing a variety of analyte such as ionic species, small molecules, macromolecules and biomacromolecules. | $65 | Add To Cart |
| MC_0010018 | MspA-M | The plasmid MspA-M is designed for the preparation of a mutant Mycobacterium smegmatis porin A (MspA) nanopore, which is engineered with a methionine in the site of 91. Six extra histidine tag are designed for the followup purification using nickel affinity chromatography. MspA is a funnel-shaped, octameric channel protein which measures ~1.2 nm in diameter at its narrowest restriction, and its structure has been characterized using X-ray crystallography (PDB ID: 1uun). With a single, geometrically sharp restriction, MspA has an excellent performance in the DNA sequencing (Manrao E A, Derrington I M, Laszlo A H, et al. Nature biotechnology, 2012, 30(4): 349.; Laszlo A H, Derrington I M, Ross B C, et al. Nature biotechnology, 2014, 32(8): 829.). Rencently, the mutant of MspA (MspA-M) has been reported as a single-molecule nanoreactor for the detection of tetrachloroaurate(III) ions. Its sensing performance is superior to α-hemolysin (α-HL), which indicates that MspA is advantageous for single molecule chemistry investigations and has applications as a hybrid biological nanopore with atomic adaptors (Cao J, Jia W, Zhang J, et al. Nature Communications, 2019, 10(1): 1-11.). | $65 | Add To Cart |
Latest Newsletters in 2020
- Can molecular nanomachines solve the problem of multi-drug-resistant pathogens?
- Are we entering an era of ternary vector system for genome editing in plants?
- Electrode-free nanopore sensing by DiffusiOptoPhysiology
Become a Cloud Scientist on MolecularCloud
Shine a light on your research and connect with the world