New direction of CD25-tumor immunotherapy
1. What is CD25?
The functional IL-2 receptor (IL-2R) consists of three parts, namely the α chain (IL2Rα, also known as CD25), the β chain (IL1Rβ, also known as CD122), and the γ chain (IL2Rγ, also known as CD 132). These three subunits are integral to the function and affinity of IL-2R. Quiescent T cells, B cells, and monocytes rarely express CD25, which is expressed at high levels in Tregs but not in Teff cells. Eliminating or reversing the immunosuppressive effects of Tregs is key to tumor immunotherapy. CD25 is a potential target for achieving Treg depletion, and its role in the tumor microenvironment (TME) and peripheral microenvironment has received extensive attention.
2. Nanobodies and their advantages
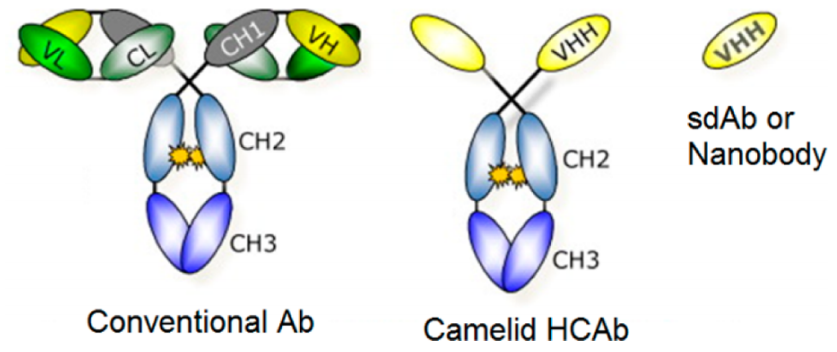
Only heavy chain antibodies (HcAbs) have been identified in camelidae. Compared to conventional IgG, these antibodies lack the CH1 domain and do not have light chains. The specificity of HcAb depends only on a heavy variable field called VHH. Recombinant production of VHH produces fragments called single-domain antibodies (sdAbs), or nanobodies. Due to their high sequence identity with human VH (family 3), nanobodies are expected to exhibit low immunogenicity in human treatments and are readily humanized, with the European Medicines Agency (EMA) approving the first therapeutic nanobody, caplacizumab. The CDR3 loop of nanobodies is typically longer than conventional VH, allowing binding to unconventional epitopes such as protein cleavages. In addition, structural studies have determined that nanobodies generally have greater complementary site diversity, involving amino acids in variable loops and framework regions. Nanobodies also have good solubility and stability to pH and temperature.
Nanobodies can also be used to design larger molecules with multiple valence states or specificities and can be easily combined with imaging agents or drug delivery systems.
3. CD25 signaling pathway and expression in tumors
When IL-2 binds specifically to IL2Rα cells, it promotes heterodimerization of IL2Rβ and IL2Rγ. Slight structural changes in IL-2 promote the formation of IL2Rα/β/γ and IL-2 tetramers, and trigger the JAK/STAT5, PI3K/Akt/mTOR and MAPK signaling pathways, which play important roles in cell growth, survival, differentiation, and immunity.
Figure 1: Crystal structure and IL-2R-mediated signaling pathway of a quaternary IL-2-IL-2R complex
CD25 is a marker of Tregs and is highly expressed in most Tregs. If the ratio of Teff/Treg cells is high, overactivation of Teff cells or excessive reduction of Treg cells will lead to autoimmune diseases. Conversely, if the proportion of Teff/Treg cells is low, anti-tumor immunity is reduced. CD25 is expressed at low levels in most solid tumors. However, it is expressed at high levels in Treg cells, which normally infiltrate into solid tumors.
Figure 2: The relationship between Treg and the tumor
4. Nanobodies as carriers for cytokine delivery
IL-2, a cytokine involved in the activation and proliferation of immune cells, was the first immunotherapy approved for cancer as early as 1992. However, while cytokines have strong antitumor potency, the main limitations are off-target activation and associated toxicity. The main strategy to circumvent this limitation is gene transfer to produce cytokines locally, or antibody-directed cytokines (immune cytokines). Both IL-2-nanobodies and IFN-γ-nanobodies work synergistically with anti-TAA antibodies to inhibit tumor growth. Immune infiltration analysis showed an increase in CD8+ count, but IL-2 constructs also showed Tregs proliferation.
5. CD25-targeted drugs
At present, drugs targeting CD25 for tumor treatment have been developed based on two methods, first, recombinant IL-2, mutant IL-2, receptor-biased IL-2 and anti-CD25 monoclonal antibodies can be used as immunomodulators to regulate IL-2 signaling, thereby affecting tumor occurrence and progression; Second, cytotoxins are delivered to CD25+ cells to kill tumors. These CD25+ cells can be tumor cells or immune cells that regulate tumor cell survival.
Figure 3: IL-2R-targeted biologic drugs currently on the market or in clinical trials
6. KMD Bioscience can provide high-quality alpaca nanobody discovery services
Compared with traditional monoclonal antibodies, the production of nanobodies has great advantages. It can be summarized as immune alpaca obtaining antibody genes and phage display to obtain the antibody sequence of interest. It is mainly divided into four steps: alpaca immunity, phage library construction, antibody library screening, antibody expression and functional verification.
6.1 Advantages in the production of nanobodies
Compared with traditional monoclonal antibodies, the production of nanobodies has great advantages. It can be summarized as immune alpaca obtaining antibody genes and phage display to obtain the antibody sequence of interest. It is mainly divided into four steps: alpaca immunity, phage library construction, antibody library screening, antibody expression and functional verification.
6.1.1 Alpaca immunization
Select the appropriate age alpaca→ prepare antigen→ immunize alpaca→ blood collection to measure titer→ PBMC cell isolation
6.1.2 Phage library construction
RNA extraction→ cDNA reverse transcription synthesis→ PCR amplification→ ligation vector and electro conversion → bacterial library/phage library establishment
6.1.3 Antibody library screening
The commonly used screening methods are solid phase screening, magnetic bead and cell screening, and KMD Bioscience can provide customized screening systems according to customer needs.
6.1.4 Antibody expression and functional verification
Mammalian expression vectors were constructed for expression → per-nickel column purification → affinity determination
Figure 4: The picture of Fc-VHH nanobody antibody expression result
CD25 has emerged as a potential therapeutic target with preclinical/clinical application prospects for immunotherapy. Various types of drugs against CD25 have been extensively studied. Due to their small molecular weight, nanobodies are more likely to penetrate tumors, and as a carrier for cytokine delivery, IL-2-nanobodies work synergistically to inhibit tumor growth, providing new ideas for cancer treatment.
- Like (1)
- Reply
-
Share
About Us · User Accounts and Benefits · Privacy Policy · Management Center · FAQs
© 2026 MolecularCloud




