How is CRISPR used in cancer research?
The first trial in the United States to test a CRISPR-made cancer therapy was launched in 2019 at the University of Pennsylvania. The study, funded in part by NCI, is testing a type of immunotherapy in which patient's’ own immune cells are genetically modified to better “see” and kill their cancer.
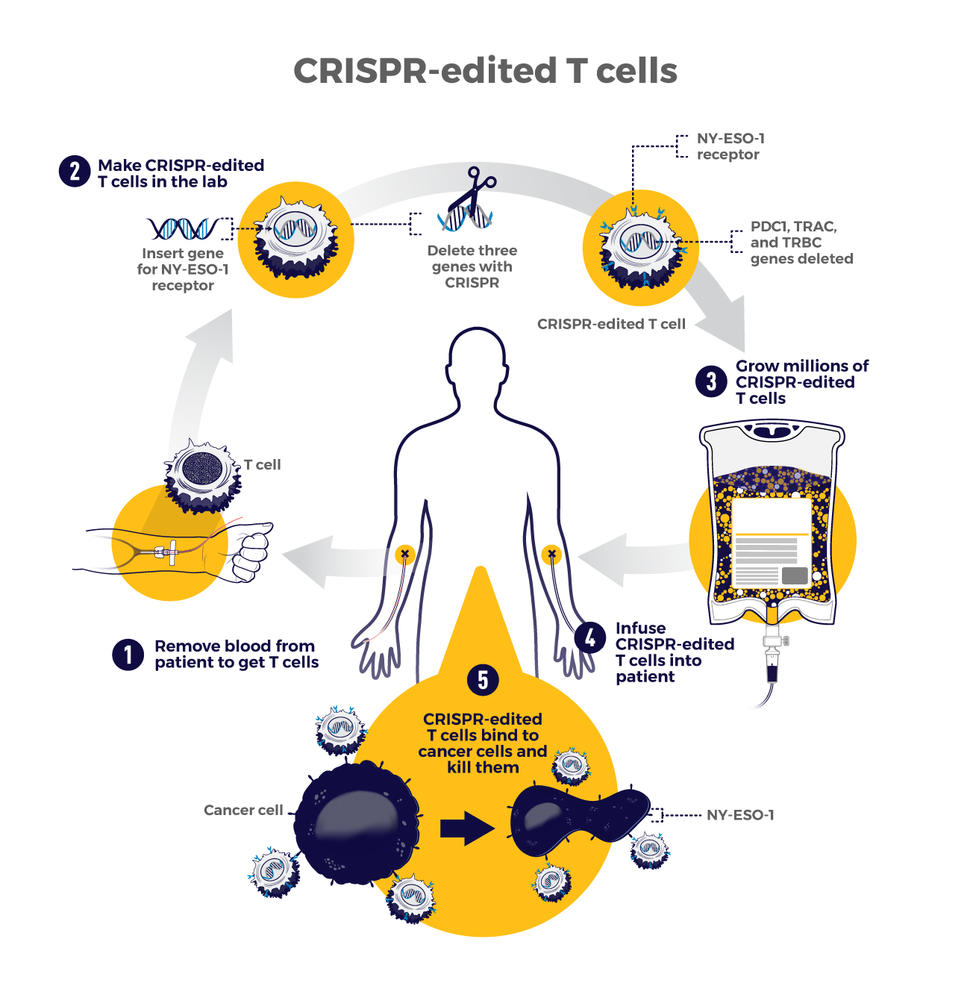
The therapy involves making four genetic modifications to T cells, immune cells that can kill cancer. First, the addition of a synthetic gene gives the T cells a claw-like protein (called a receptor) that “sees” NY-ESO-1, a molecule on some cancer cells.
Then CRISPR is used to remove three genes: two that can interfere with the NY-ESO-1 receptor and another that limits the cells’ cancer-killing abilities. The finished product, dubbed NYCE T cells, were grown in large numbers and then infused into patients.
“We had done a prior study of NY-ESO-1–directed T cells and saw some evidence of improved response and low toxicity,” said the trial’s leader, Edward Stadtmauer, M.D., of the University of Pennsylvania. He and his colleagues wanted to see if removing the three genes with CRISPR would make the T cells work even better, he said.
The goal of this study was to first find out if the CRISPR-made treatment was safe. It was tested in two patients with advanced multiple myeloma and one with metastatic sarcoma. All three had tumors that contained NY-ESO-1, the target of the T-cell therapy.
Initial findings suggest that the treatment is safe. Some side effects did occur, but they were likely caused by the chemotherapy patients received before the infusion of NYCE cells, the researchers reported. There was no evidence of an immune reaction to the CRISPR-edited cells.
Only about 10% of the T cells used for the therapy had all four of the desired genetic edits. And off-target edits were found in the modified cells of all three patients. However, none of the cells with off-target edits grew in a way that suggested they had become cancer, Dr. Stadtmauer noted.
The treatment had a small effect on the patients’ cancers. The tumors of two patients (one with multiple myeloma and one with sarcoma) stopped growing for a while but resumed growing later. The treatment didn't work at all for the third patient.
It's exciting that the treatment initially worked for the sarcoma patient because “solid tumors have been a much more difficult nut to crack with cellular therapy," Dr. Stadtmauer said. "Perhaps [CRISPR] techniques will enhance our ability to treat solid tumors with cell therapies.”
Although the trial shows that CRISPR-edited cell therapy is possible, the long-term effects still need to be monitored, Dr. Stadtmauer continued. The NYCE cells are “safe for as long as we’ve been watching [the study participants]. Our plan is to keep monitoring them for years, if not decades,” he said.
Reference: https://www.cancer.gov/news-events/cancer-currents-blog/2020/crispr-cancer-research-treatment#:~:text=The%20first%20trial%20in%20the,see%E2%80%9D%20and%20kill%20their%20cancer.
- Like (8)
- Reply
-
Share
About Us · User Accounts and Benefits · Privacy Policy · Management Center · FAQs
© 2025 MolecularCloud