Catalysts for Epoxidation
Epoxidation is a very important organic reaction, which refers to the reaction of an olefin to form an epoxide under the action of a reagent. Organic peracids such as peracetic acid, peroxybenzoic acid, m-chloroperoxybenzoic acid and the like are epoxidizing agents commonly used in the laboratory. The epoxidation reaction conditions are mild and the yield is high, so it is widely used in organic synthesis. The general formula of the epoxidation reaction is shown in figure 1.
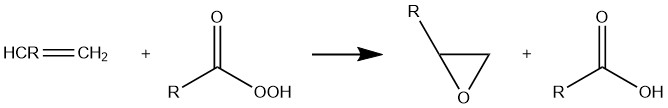 Figure 1. The schematic diagram of the mechanism of epoxidation reaction
Figure 1. The schematic diagram of the mechanism of epoxidation reaction
Applications
The most widely used epoxides in the industry are ethylene oxide and propylene oxide, which are produced at a scale of about 150,000 tons/year and 3 million tons/year, respectively. For example, ethylene oxide is a relatively common epoxy compound and is widely used to produce detergents and surfactants used in people's life by ethoxylation. Ethylene glycol hydrolysis can also give ethylene glycol. In addition, it is also used for the disinfection of medical devices and materials. The reaction of the epoxide with the amine is the basis for the formation of epoxy gums and structural materials. Triethylenetetramine (TETA) is a typical amine hardener.
In addition, epoxy compounds are mainly used in the production of various nonionic surfactants, as well as in the production of plasticizers, lubricants, rubber and plastics. They are widely used in dyeing, electronics, medicine, pesticides, textiles, paper, automotive, oil mining and refining and many other fields.
Classification
There are currently many classifications of epoxidation reactions, such as Heterogeneously catalyzed oxidation of alkenes, Olefin oxidation using organic peroxides and metal catalysts, Olefin peroxidation using peroxycarboxylic acids, Homogeneously catalysed asymmetric epoxidations, Intramolecular SN2 substitution, Nucleophilic epoxidation and Biosynthesis. There are two main forms of epoxidation.
- Indirect epoxidation: The olefins are first prepared as halohydrins, and then reused or de-cyclized.
- Direct epoxidation: 1 Alkene Air Oxidation Process: Lower olefins are oxidized in gas phase by air in the presence of a catalyst. 2 Olefinic Peroxyacid Oxidation Method: organic peroxyacid can be prepared from carboxylic acid first. The olefin and a certain amount of peroxyacid are subjected to a low temperature reaction (0℃ to room temperature) in an anhydrous inert organic solvent with an acid catalyst (strongly acidic ion exchange resin).
The improved method is called "in situ epoxidation method". It is to dissolve the olefins in glacial acetic acid, add a small amount of acid catalyst, and then add them in portions at 60-70℃, and then react the peroxy vinegar formed in the reaction system.
Catalysts
With the advancement of technology, the types of catalysts for epoxy reaction are also increasing. The common catalysts for epoxy reaction are: metal porphyrin catalysts, titanium silicon molecular sieves, methyl antimony trioxide, and single metal salt catalysts (such as manganese salt catalyst), heteropoly acid compound catalyst, and the like.
References
- W.R.Thiel. (1997).‘Metal catalyzed oxidations. Part 5. Catalytic olefin epoxidation with seven-coordinate oxobisperoxo molybdenum complexes: a mechanistic study’. Journal of Molecular Catalysis A: Chemical. 117 : 449-454.
- Katsuki, T. (1980). ‘The first practical method for asymmetric epoxidation’. J. Am. Chem. Soc. 102 (18): 5974-5976.
- C.J.Thibodeaux. (2012). ‘Enzymatic Chemistry of Cyclopropane, Epoxide, and Aziridine Biosynthesis’. Chem. Rev. 112: 1681-1709.
- Like
- Reply
-
Share
About Us · User Accounts and Benefits · Privacy Policy · Management Center · FAQs
© 2025 MolecularCloud



