It has now been nearly 40 years since the human immunodeficiency virus (HIV) was discovered. Based on the CDC, an estimated 1.2 million individuals were infected with HIV in the USA by 2018. Worldwide, 37.9 million individuals lived with HIV by 2018, as estimated by the United Nations on HIV/AIDS (Illanes-Alvarez et al. 2021). Antiretrovirals currently represent the primary therapy for HIV, which inhibits viral replication and allows immune function recovery. Nevertheless, there are no therapies enabling viral eradication.
Since 1987 multiple HIV vaccine candidates have been developed and clinically tested. Some vaccine candidates have been abandoned in the absence of efficacy. Other HIV vaccine leads are currently at various stages of clinical testing (Deeks et al. 2015). For example, Phase 2 and 3 studies (Imbokodo and Mosaico) sponsored by Janssen Vaccines & Prevention B.V. evaluate the efficacy of a mosaic vaccine containing antigens from several HIV subtypes. More recently, positive results were reported from a Phase 1 clinical trial evaluating a new vaccine (eOD-GT8 60mer) candidate’s safety and ability to induce immunogenicity. The study, sponsored by the International AIDS Vaccine Initiative (IAVI), is the first step in developing an immunization strategy to promote the production of anti-HIV “broadly neutralizing antibodies.”
We are very familiar with “neutralizing antibodies,” which have been at the center of the ongoing COVID-19 pandemic. For SARS-CoV-2, neutralizing antibodies target the Spike protein inhibiting viral cell-entry. Recent studies support the critical role of anti-SARS-CoV-2 neutralizing antibodies in protective immunity (Wajnberg et al. 2020). Similarly, during HIV infections, the envelope spike protein is targeted by neutralizing antibodies. Early antibody-neutralizing responses may not effectively inhibit viral replication due to the high mutational rate facilitating viral escape. However, investigators have recognized that antibodies with broad neutralizing activity may develop over the course of HIV infections. Broadly neutralizing antibodies target conserved viral surface epitopes shared by a wide range of HIV subtypes. Therefore vaccine strategies promoting this type of antibody are expected to confer effective protection (Su et al. 2019).
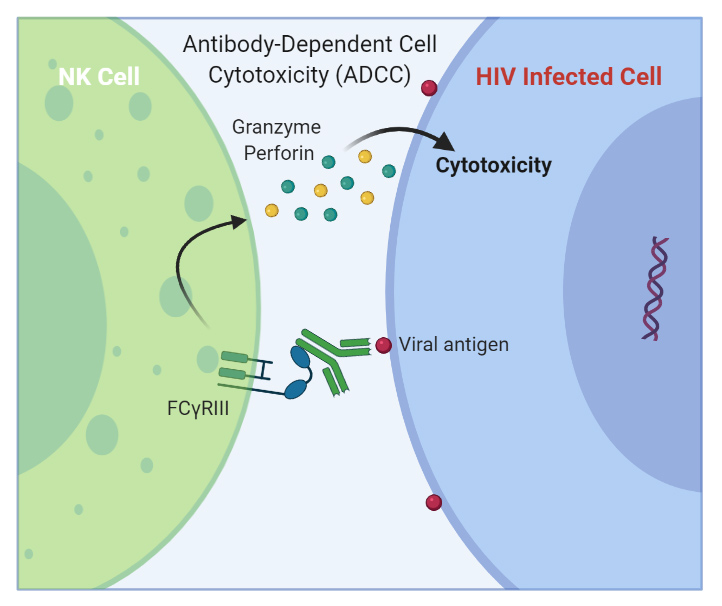
In ADCC, antibodies recognize specific epitopes in HIV-infected cells and interact via their Fc domain with specific Fcγ receptors expressed by effector cells (e.g., N.K. cells), triggering the release of perforin and granzymes and leading to the elimination of infected cells.
Beyond antibody viral neutralization, investigators believe that Fc-mediated effector functions also play a role in protective immunity to HIV. Fc-mediated effector functions include antibody dependent-cellular phagocytosis (ADCP), -cellular cytotoxicity (ADCC), and –cellular virus inhibition (ADCVI), among others. For example, ADCC has been implicated in reduced mother-to-infant HIV transmission and partial protection conferred by a clinically tested vaccine candidate (RV144 vaccine trial) (Su et al. 2019, Doepker et al. 2021).
Antibodies with ADCC function may be a critical component of vaccine-induced protective immunity against HIV. Therefore, investigators are keen to understand the origin, development, and properties of antibodies supporting ADCC. To this end, recently, Doepker and colleagues took a closer look at how precisely these antibodies come about by leveraging targeted sequencing analysis (NGS) and computational approaches.
They focused on several monoclonal antibodies previously isolated from an HIV-infected individual, which were primarily non-neutralizing and had strong ADCC function. Sequencing of samples obtained at different times, pre and post-HIV infection, allowed them to understand the origins or infer potential antibody “naïve precursors” and intermediates, giving rise to the identified antibodies. By following this approach, Doepker et al. identified key antibody domains and regions where evolution through specific mutations enacted changes supporting ADCC activity. Overall, investigators are hopeful that these findings will help support immunogen design and vaccine strategies aiming to induce antibodies with potent ADCC function.
Reference
Deeks, S. G., Overbaugh, J., Phillips, A. & Buchbinder, S. HIV infection. Nat. Rev. Dis. Prim. (2015) doi:10.1038/nrdp.2015.35.
Doepker, L. E. et al. Development of antibody-dependent cell cytotoxicity function in HIV-1 antibodies. Elife. 2021 Jan 11;10:e63444. doi: 10.7554/eLife.63444.
Illanes-álvarez, F., Márquez-Ruiz, D., Márquez-Coello, M., Cuesta-Sancho, S. & Girón-González, J. A. Similarities and differences between hiv and sars-cov-2. Int. J. Med. Sci. (2021) doi:10.7150/ijms.50133.
Su, B. et al. Update on Fc-Mediated Antibody Functions Against HIV-1 Beyond Neutralization. Frontiers in Immunology(2019) doi:10.3389/fimmu.2019.02968.
Wajnberg, A. et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science (80-. ). (2020) doi:10.1126/science.abd7728.